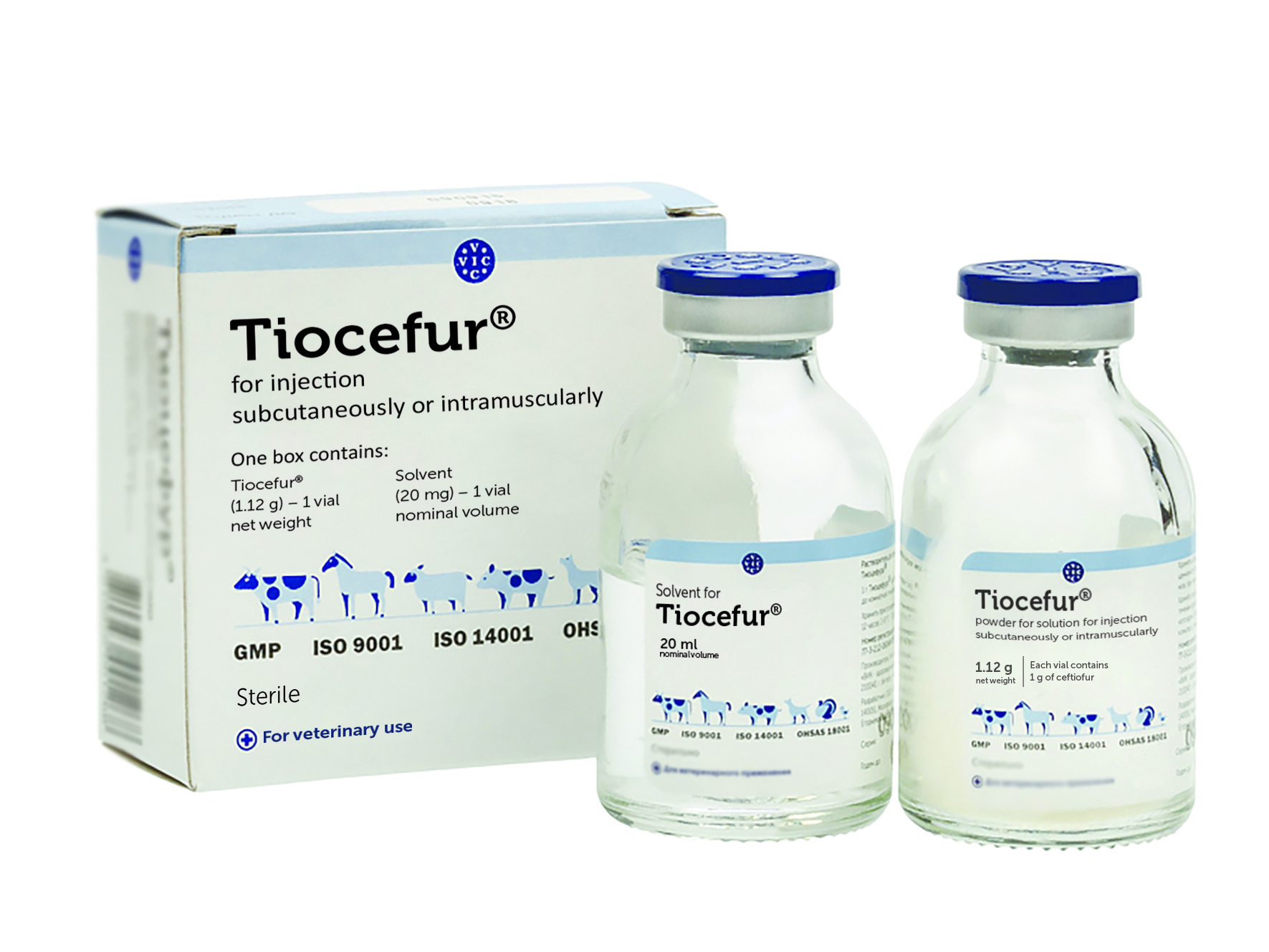
Tiocefur®
Solution for injection intramuscular and subcutaneous administration





Specification
Ceftiofur is effective against gram-positive and gram-negative
bacteria, including strains producing beta-lactamase and some
anaerobic bacteria, Pasteurella (Mannheimia) haemolytica, Pasteurella
multocida, Haemophilus somnus, Haemophilus parasuis, Streptococcus
zooepidemicus, Streptococcus suis, Actinobacillus pleuropneumoniae,
Escherichia coli, Salmonella cholerasuis, Salmonella typhimurium,
Fusobacterium necrophorum, Bacteroides melaninogenicus
(Porphiromonas assacharolytica), Actynomyces pyogenes, Staphylococcus
spp., Klebsiella spp., Citrobacter spp., Bacillus spp., Proteus spp.
Depending on animal species the therapeutic concentration
persists for 20 hours after parenteral administration. The maximum
concentration is detected in an hour after administration. Tiocefur®
is mainly excreted by the kidneys.
Indications
• treatment of respiratory diseases of bacterial etiology and necrobacillosis in cattle
• treatment of respiratory diseases of bacterial etiology in cattle, pigs and horses
• treatment of respiratory and genitourinary diseases in dogs caused by ceftiofur-sensitive bacteria
• prevention of bacterial diseases in chickens and turkey poults.
Composition
One box contains a vial with 1 g or 4 g of ceftiofur sodium and a vial with 20 ml or 80 ml of solvent.
Dosage
Tiocefur® powder is dissolved in the solvent preheated to room temperature: 1 g of the powder per 20 ml of the solvent, 4 g of the powder per 80 ml of the solvent (the concentration of the resulting solution is 50 mg of ceftiofur sodium per 1 ml). The resulting solution is stored at 20-25 °C for no longer than 12 hours, or at 2-8 °C for no longer than 7 days, or at 4-18 °C for no longer than 50 days.
Tiocefur® is administered intramuscularly or subcutaneously to cattle, sheep and goats, intramuscularly to pigs and horses, subcutaneously to dogs, subcutaneously to poultry at the following doses:
• cattle – 1-2 ml of the solution per 50 kg bw (1-2 mg of ceftiofur per 1 kg), but not more than 15 ml per one injection site, for 3-5 days;
• sheep and goats – 0.1-0.2 ml of the solution per 5 kg bw (1-2 mg of ceftiofur per 1 kg), but not more than 5 ml per one injection site, for 3-5 days;
• pigs – 0.3-0.5 ml of the solution per 5 kg bw (3-5 mg of ceftiofur per 1 kg), but not more than 10 ml per one injection site, for 3 days;
• horses – 2-4 ml of the solution per 50 kg bw (2-4 mg of ceftiofur per 1 kg), but not more than 10 ml per one injection site, until recovery, but for no longer than 10 days;
• dogs – 0.2-0.4 ml of the solution per 5 kg bw (2-4 mg of ceftiofur per 1 kg), but not more than 5 ml per one injection site, until recovery, but for no longer than 10 days;
poultry:
one-day old chickens – once at 0.1-0.2 mg of ceftiofur per chicken; one-day old turkey poults – once at 0.2-0.5 mg of ceftiofur per chicken.
Tiocefur® is administered to poultry subcutaneously into the neck region at a dose of 0.2 ml.